Kinetic Isotope Effect
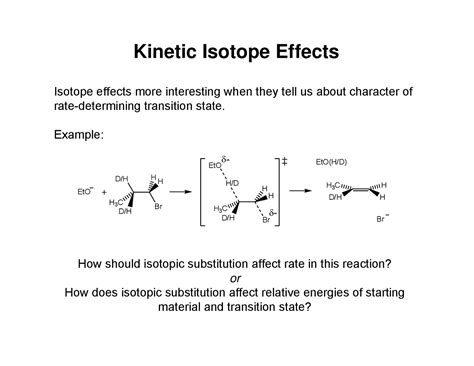
Introduction to Kinetic Isotope Effect
The kinetic isotope effect is a phenomenon that has garnered significant attention in the fields of chemistry and physics. It refers to the change in the rate of a chemical reaction when one of the atoms in the reactants is replaced with a different isotope of the same element. This effect is particularly useful in understanding the mechanisms of chemical reactions and has numerous applications in fields such as nuclear chemistry, geochemistry, and environmental science. In this blog post, we will delve into the concept of the kinetic isotope effect, its causes, and its applications.
Causes of Kinetic Isotope Effect
The kinetic isotope effect arises due to the differences in the masses of the isotopes involved. When an atom in a molecule is replaced with a heavier isotope, the vibrational frequency of the molecule decreases. This decrease in vibrational frequency leads to a higher activation energy for the reaction, resulting in a decrease in the reaction rate. Conversely, when a lighter isotope is used, the vibrational frequency increases, leading to a lower activation energy and an increase in the reaction rate.💡 Note: The kinetic isotope effect is most pronounced when the reaction involves the breaking or forming of bonds to the isotopically substituted atom.

Types of Kinetic Isotope Effects
There are two primary types of kinetic isotope effects: primary and secondary. A primary kinetic isotope effect occurs when the bond to the isotopically substituted atom is broken or formed in the rate-determining step of the reaction. This type of effect is typically large and can be used to determine the mechanism of a reaction. A secondary kinetic isotope effect, on the other hand, occurs when the bond to the isotopically substituted atom is not broken or formed in the rate-determining step, but the substitution still affects the reaction rate.
Applications of Kinetic Isotope Effect
The kinetic isotope effect has numerous applications in various fields. Some of the most significant applications include: * Nuclear chemistry: The kinetic isotope effect is used to study the mechanisms of nuclear reactions and to determine the reaction rates. * Geochemistry: The kinetic isotope effect is used to study the formation of minerals and rocks, and to determine the conditions under which they formed. * Environmental science: The kinetic isotope effect is used to study the fate and transport of pollutants in the environment. * Pharmaceuticals: The kinetic isotope effect is used to study the metabolism of drugs and to develop new pharmaceuticals.
Experimental Methods for Measuring Kinetic Isotope Effects
There are several experimental methods for measuring kinetic isotope effects, including: * Mass spectrometry: This method involves measuring the ratio of the reactants and products using a mass spectrometer. * Nuclear magnetic resonance (NMR) spectroscopy: This method involves measuring the NMR spectra of the reactants and products. * Gas chromatography: This method involves separating the reactants and products using a gas chromatograph.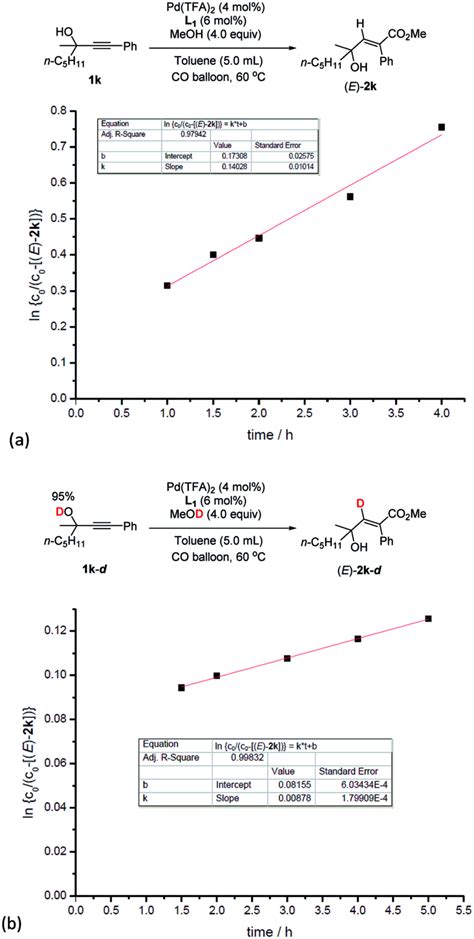
| Method | Description |
|---|---|
| Mass spectrometry | Measures the ratio of reactants and products |
| NMR spectroscopy | Measures the NMR spectra of reactants and products |
| Gas chromatography | Separates reactants and products |

Theory Behind Kinetic Isotope Effect
The theory behind the kinetic isotope effect is based on the concept of transition state theory. According to this theory, the reaction rate is determined by the energy of the transition state, which is the highest energy state along the reaction coordinate. The kinetic isotope effect arises due to the differences in the zero-point energies of the reactants and products, which affect the energy of the transition state.In summary, the kinetic isotope effect is a powerful tool for understanding the mechanisms of chemical reactions and has numerous applications in various fields. By understanding the causes and applications of the kinetic isotope effect, researchers can gain valuable insights into the behavior of molecules and develop new methods for studying chemical reactions.
The main points to take away from this discussion are the concept of the kinetic isotope effect, its causes, and its applications. The kinetic isotope effect is a phenomenon that occurs when the rate of a chemical reaction is affected by the replacement of an atom with a different isotope. The effect is caused by the differences in the masses of the isotopes involved and can be used to determine the mechanism of a reaction. The kinetic isotope effect has numerous applications in fields such as nuclear chemistry, geochemistry, and environmental science.

What is the kinetic isotope effect?
+
The kinetic isotope effect is a phenomenon that occurs when the rate of a chemical reaction is affected by the replacement of an atom with a different isotope.

What are the causes of the kinetic isotope effect?
+
The kinetic isotope effect is caused by the differences in the masses of the isotopes involved, which affect the vibrational frequency of the molecule and the energy of the transition state.

What are the applications of the kinetic isotope effect?
+
The kinetic isotope effect has numerous applications in fields such as nuclear chemistry, geochemistry, environmental science, and pharmaceuticals.

